shopping_basket
Efficient Lipid Nanoparticles: The Microfluidic Approach to Precision Medicines
- Expertise
- Jul 16, 2024
- Reading time: 9 minutes

Remember those groundbreaking COVID-19 vaccines? They've got a secret weapon: lipid nanoparticles (LNPs). These microscopic marvels were the unsung heroes behind the Pfizer-BioNTech and Moderna vaccines. But here's the kicker - LNPs aren't just for vaccines. They're now the hottest trend in drug delivery, promising to transform how we treat everything from rare genetic disorders to cancer. Curious about the science behind these tiny powerhouses? Wondering how they might change your future healthcare? Get ready to discover why LNP’s are making such a visible impact on our health.
Understanding LNPs
Picture this: you've got a precious package (like mRNA) that needs to reach a specific destination in your body. But there's a problem - your body's defenses are ready to destroy it. LNPs are stable, biocompatible particles with a bilayer lipid shell consisting of four components: cationic/ionizable lipids, PEG, auxiliary lipids, and cholesterol, that encompass an aqueous core in which they can carry mRNA to protect it from destruction [1]. These fat-like substances are recognized as friendly, making LNPs act like stealthy vehicles, sneaking past your immune system and fusing with cell membranes to deliver their cargo right where it's needed.


These nanoparticles have become essential in modern medicine. Some of the key applications of LNPs are mRNA vaccines, gene therapy, cancer and protein replacement therapies, as well as non-medical applications in cosmetics and the food industry. By precisely targeting specific cells, LNPs ensure that treatments reach their intended destinations, maximizing effectiveness while minimizing side effects
Microfluidic Techniques for LNP Production
LNPs can be produced using different microfluidic techniques, each offering distinct advantages. Let's break down the two main approaches as highlighted by Shepherd et al. [2]
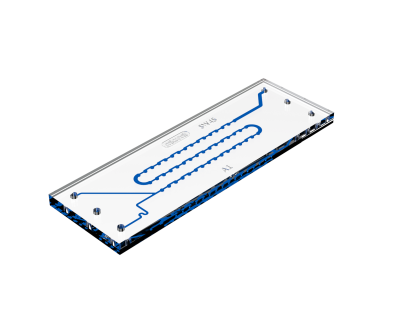
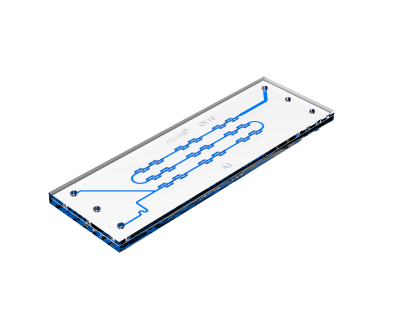
1. Continuous Flow Microfluidics
Continious flow involves mixers with a steady flow of two or more solutions through microchannels, allowing for the formation of nanoparticles through laminar flow, diffusion and reaction processes. These techniques offer precise control over nanoparticle size and composition by adjusting flow rates and channel dimensions and are scalable for higher volume production. Which makes it a suitable technique for pharmaceutical applications.
Ideal for: Stable laminar flow with extended mixing periods.
Mechanism: Diffusion-based mixing through a serpentine channel, allowing for precise monitoring.
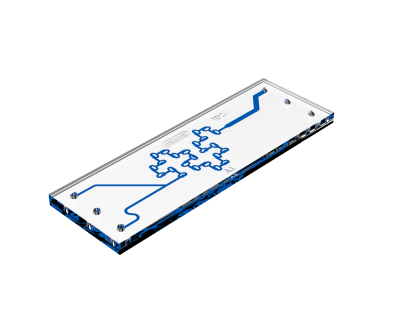
- T-shaped channel
- Serpentine channel (the larger versions with 3-inlet can also be used in a flow focussing mode)
- H-shaped channel


2. Chaotic advection mixers
The chaotic advection mixers use complex microchannel designs to enhance mixing through chaotic flow patterns, which optimizes the efficiency of nanoparticle formation. The particle formation and encapsulation efficiency contribute to the suitability of this technique for personalized medicine.
Ideal for: Creating chaotic advection in laminar flow regimes.
Mechanism: Special features create imbalances in the flow, enhancing mixing fand reducing mixing time.


Choosing the right technique depends on your needs. Micronit offers a diverse catalog of micromixer chips, each designed for specific mixing requirements. Our standard mixer chips are crafted from Borosilicate glass using wet etching or laser ablation techniques. Are you a researcher looking for perfect particles, or a manufacturer aiming to produce millions of doses? Each method has its sweet spot. Micronit is able to help develop systems to generate LNP’s for your need.
Benefits of Using Microfluidics for LNP Production
Microfluidic technology offers several significant advantages in the production of Lipid Nanoparticles (LNPs), contributing to advancements in nanomedicine:
- Precise Size Control: Microfluidics enables exceptional control over LNP size distribution and polydispersity index (PDI). This precision is crucial for optimizing pharmacokinetics and cellular uptake, directly impacting therapeutic efficacy.
- Reproducibility: The laminar flow characteristics in microfluidic channels ensure consistent mixing conditions, resulting in highly reproducible LNP formulations. This is essential for batch-to-batch consistency in pharmaceutical production.
- Rapid Prototyping: Microfluidic platforms facilitate rapid screening of formulation parameters, accelerating the development cycle of new LNP-based therapeutics.
- Scalability: Many microfluidic techniques offer seamless scalability from preclinical to industrial production volumes, maintaining consistent physicochemical properties across scales.
- Process Control: Real-time monitoring and precise control of flow rates, temperatures, and mixing ratios in microfluidic systems allow for fine-tuning of LNP characteristics.
- Encapsulation Efficiency: The rapid mixing achieved in microfluidic devices often results in higher encapsulation efficiencies of payload molecules (e.g., mRNA, siRNA) compared to conventional methods.
These advantages collectively contribute to the production of higher quality LNPs with improved batch-to-batch consistency, accelerating the translation of LNP-based therapeutics from laboratory to clinic.
Applications of LNPs Produced via Microfluidics
Recent advancements in microfluidic production of Lipid Nanoparticles (LNPs) have led to significant breakthroughs in various biomedical fields. Here are the applications ordered by their current prevalence and impact:
- Vaccine Development: The success of LNP-based COVID-19 vaccines has catapulted this application to the forefront of LNP research and development [3]. Microfluidic techniques ensure the production of uniformly sized LNPs, critical for consistent vaccine delivery and immune response. This approach is now being widely adapted for other infectious diseases, potentially accelerating vaccine development timelines and revolutionizing global health initiatives.
- Cancer Immunotherapy: LNPs are rapidly emerging as crucial vectors in mRNA-based cancer treatments [4]. By efficiently delivering mRNA encoding tumor antigens, cytokines, and therapeutic proteins, these nanoparticles are enhancing immune responses against cancer cells. This technology is opening new avenues in cancer vaccine development and personalized immunotherapies, making it a highly active area of research and clinical trials.
- Personalized Medicine: Microfluidic production of LNPs enables rapid and scalable creation of personalized formulations. This allows for tailoring treatments to individual patient profiles based on genetic markers or specific disease characteristics, particularly beneficial in oncology and rare genetic disorders [5]. While still emerging, we see this application has significant potential to transform patient care in the future.
These advancements underscore the transformative potential of microfluidic-produced LNPs across various medical fields. As research progresses, we anticipate to help customers develop new microfluidic based solutions in this field.
Microfluidics & LNPs: Challenges and Future Directions
Although microfluidics has revolutionized LNP production, particularly for mRNA vaccines, significant challenges remain. The intricate lab-on-a-chip systems require precise expertise. Additionally, integrating microfluidic systems, adhering to regulations, and implementing robust quality controls are crucial for applications like drug delivery.
Despite these challenges, the future of microfluidic LNP production is promising. Lipid nanoparticles play a crucial role in the efficient and targeted delivery of genetic therapies, personalized treatments, and effective vaccines.
Micronit, as a leading partner in microfluidics, is deeply involved in these promising developments. We assist clients in developing new LNP systems, offering expertise and support in scaling up processes to ensure the efficiency and consistency needed for commercial production.
Sources:
[1] Mashima, R., & Takada, S. (2023). Lipid nanoparticles: A novel gene delivery technique for clinical application. Pharmaceutics, 15(4), 1127. http://dx.doi.org/10.3390/pharmaceutics15041127
[2] Shepherd SJ, Issadore D, Mitchell MJ. Microfluidic formulation of nanoparticles for biomedical applications. Biomaterials. 2021 Jul;274:120826. doi: 10.1016/j.biomaterials.2021.120826. Epub 2021 Apr 26. PMID: 33965797; PMCID: PMC8752123. https://dx.doi.org/10.1016/j.biomaterials.2021.120826
[3] Buschmann, M. D., Carrasco, M. J., Alishetty, S., Paige, M., Alameh, M.-G., & Weissman, D. (2021). Nanomaterial delivery systems for mRNA vaccines. Vaccines, 9(1), 65. DOI: 10.3390/vaccines9010065
[4] Sun H, Zhang Y, Wang G, Yang W, Xu Y. mRNA-Based Therapeutics in Cancer Treatment. Pharmaceutics. 2023 Feb 13;15(2):622. doi: 10.3390/pharmaceutics15020622. PMID: 36839944; PMCID: PMC9964383.
[5] Pardi, N., Hogan, M. J., Porter, F. W., & Weissman, D. (2018). mRNA vaccines—a new era in vaccinology. Nature Reviews Drug Discovery, 17(4), 261-279. DOI: 10.1038/nrd.2017.243